Reproduction and dispersal
Two forms of reproduction can be found amongst living organisms - sexual and asexual. It is by the mixing of genes from two individuals, via sexual reproduction, that genetic diversity is effected, whereas in asexual reproduction there is no such mixing of genes. Both sexual and asexual reproduction can be found amongst the lichens. When talking of plants (or lichens, which were once thought of as plants) asexual reproduction is commonly called vegetative reproduction. Though lichens, as a whole, may reproduce both sexually or vegetatively, there are species in which both types of reproduction may be common but also species where one type is rare or even unknown. In each form of reproduction propagules of some sort are produced and dispersed and there is a separate page dealing with PROPAGULE DISPERSAL. The SEXUAL VS. VEGETATIVE page gives some general comments about the two means of reproduction.
When talking about lichen reproduction two fundamental questions arise:
What does it mean to talk of sexual reproduction in lichens?
What is an individual lichen?
The next two sections will look at those questions.
What does it mean to talk of sexual reproduction in lichens?
After all, no lichen is an individual organism but an association between two (or more) organisms. In fact in lichens only the fungal partners may reproduce sexually. To say that lichens may reproduce sexually is really shorthand for "the fungal partners within lichens may reproduce sexually". A number of photobiont species found in lichens can be found free-living and could then reproduce sexually but within a lichen sexual reproduction of the photobiont is suppressed.
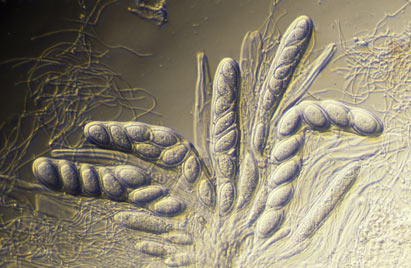
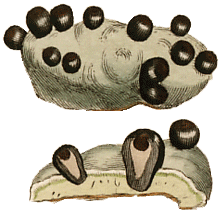
The sexually reproducing lichens are either ascomycetes or basidiomycetes. Ascomycetes produce their sexual propagules (called ascospores) within microscopic organs called asci and basidiomycetes produce their sexual propagules (called basidiospores) on microscopic organs called basidia. Often ascospores or basidiospores are simply called spores, especially where there is no need to differentiate the two or where there is no possible confusion with vegetative propagules. Individual asci or basidia cannot be seen with the naked eye but they are produced in large numbers in easily visible structures. The most commonly seen ascospore-producing structures are the apothecia, typically disc-like to cup-like and found growing from the thallus surface. The REPRODUCTIVE STRUCTURES page gives descriptions of various structures associated with the production of ascospores and the BASIDIOLICHEN page gives examples of the structures in which basidiospores are produced.
Much is known about the processes of sexual reproduction in the non-lichenized ascomycetes or basidiomycetes since it is possible to grow many of them in the laboratory, follow all steps of their life cycles and carry out mating experiments. By these means it has been possible to see how the processes of sexual reproduction are carried out in those species. Such research has shown that the sexual reproduction in the non-lichenized ascomycetes or basidiomycetes has both similarities to and significant differences from the processes organisms such as humans or plants. It has not been possible to elucidate most of the processes in the lichen mycobionts. A number of them can be grown, to some degree, in the laboratory but not to the extent that would allow a good understanding of all the processes of sexual reproduction. In this context it is worth noting that, amongst the non-lichenized ascomycetes or basidiomycetes, it is harder to study the processes in mycorrhizal species than in saprotrophic ones. In the SEXUAL REPRODUCTION CASE STUDY you can find a brief description of some aspects of sexual reproduction in non-lichenized ascomycetes and basidiomycetes and briefer comments about the lichenized fungi.
What is an individual lichen?
You know that lichens are associations between fungi and photobionts. Let's keep things simple for the moment and assume we have a thallus that contains just one photobiont species and one fungal species. The answer to the previous fundamental question revealed that sexual reproduction in lichens involves only the fungal partner so an important question is: Does a lichen thallus contain just one fungal individual? The answer is: Sometimes yes, sometimes no.
At this point it is useful to introduce the word mycelium, a fundamental concept for non-lichenized fungi. When a propagule of an ascomycete or basidiomycete germinates it would usually do so via a hyphal outgrowth which, by repeated extension and branching, would produce a network of hyphae and this network is called a mycelium. In a non-lichenized fungus this mycelium would spread through say soil, dung or wood, acquiring nutrients from the substrate but staying out of sight. In a lichenized fungus a mycelium associates with a species of photobiont and is contained within the lichen thallus, which is usually easily visible though the thalli of some crustose species may grow hidden in fissures within wood or rock.
Suppose a fungal propagule that has been released from a lichen comes to land near some free-living alga. It is irrelevant whether the propagule has been produced sexually or vegetatively. Suppose further that the propagule germinates to give rise to a mycelium that entraps some of the nearby alga and so develops a new lichen thallus. In this scenario, where all the hyphae within the thallus belong to a single mycelium that developed from one propagule, we can say that the thallus contains just a single fungal individual. Now suppose that a couple of genetically distinct fungal propagules of the same species have landed close together and near some free-living alga. Suppose both fungal propagules germinate, capture some algal cells, grow into mycelia and so give rise to two thalli but in this case the nearness of the two propagules leads to the two thalli merging. After the merger the naked eye sees just one thallus but within that thallus there are two fungal individuals - of the same species but genetically distinct. In the genus Xanthoria the ascospores are often released in groups of eight. Laboratory studies involving several Xanthoria species have shown that within a couple of days after ejection from the ascus the spores are held together by a mucilage that makes it impossible to separate the spores. Suppose several spores within the octet germinate and capture photobiont cells to generate a new thallus. The new thallus would be composed of genetically distinct fungal individuals if the spores themselves were genetically distinct![]() .
.
The emphasis has been on the fungi since the fungal partners may reproduce sexually. Nevertheless it is worth noting that when one (or more) fungal propagules land near free-living photobionts and capture them to form a new lichen thallus, the photobionts may also have been genetically diverse. Therefore a single lichen thallus, even if it contains just one species of photobiont might still show genetic diversity amongst the photobiont cells.
Vegetative reproduction
Many lichens will produce new individuals from fragments that break off from a thallus. Each fragment contains both mycobiont and photobiont cells and, in principle, functions in much the same way as a cutting taken by a gardener who wishes to propagate a particular plant. The gardener's cutting is genetically identical to the parent plant and is therefore a means of vegetative reproduction and a thallus fragment also propagates an existing genetic makeup. Fragmentation involves no special structures, though it's clear that thallus morphology plays a role since thalli with delicate growth forms are more likely to fragment than are the robust thalli. For example a number of the pendant curtain-like species that grow on trees can be torn easily by strong winds or wind-blown debris and various foliose species have thalli in which narrow, easily breakable lobes are present.
The most significant vegetative propagules are isidia and soredia. The former are small outgrowths of the thallus, up to a millimetre or so in length, which contain both fungal hyphae and photobiont cells. Broken off isidia may be transported by wind, water or animals (for example, caught on bird feet or in animal fur) and if deposited into a suitable habitat may generate new thalli. Soredia look like small powdery, granules, between about 20 and 100 micrometres in diameter, and each soredium consists of a few photobiont cells surrounded by fungal hyphae. Soredia are very easily dispersed by wind, water or animal.
The term symbiotic propagule is a general term for any sort of propagule in which both fungal and photobiont cells are present. As well as symbiotic propagules it is possible to have vegetative propagules where only one partner is present. Many lichens produce vegetative fungal propagules called conidia, often in tiny chambers called pycnidia that are embedded within a thallus but with an opening (or ostiole) to the outside through which the conidia can escape. In some circumstances photobiont cells may escape from a thallus and then function as vegetative propagules of the photobiont. Escape is especially likely in some species when the thalli are highly saturated with water.
From what was said in answer to the second fundamental question above it is clear that a lichen thallus could contain genetically distinct fungal or photobiont cells. If that were the case then vegetative propagules produced at different parts of a thallus could be genetically distinct with respect to mycobiont, photobiont or both. To be precise, it would be necessary to say that a vegetative propagule would produce a new thallus genetically identical to that part of the parent thallus that produced the propagule.
While on the subject of genetically diverse thalli it's relevant to note that the generation of such thalli need not rely on fungal propagules. In both Hypogymnia and Physcia soredia have been seen to coalesce in the early stages of the formation of new thalli. Clearly, if the soredia are genetically distinct (in one or both partners) then the new thallus also will be genetically diverse![]() .
.
An oddity - ascospore to conidium to mycelium
This short section has been put here simply to show you that the ascospore-to-mycelium path is not always direct. The ascospores of the species Vezdaea aestivalis have not been seen to produce a mycelium. Instead a spore produces a short hyphal outgrowth from which a conidium is formed and released. Such conidia give rise to extensive hyphal growth. If you consider fungi as a whole the production of conidia from sexual spores is not unique to Vezdaea aestivalis for there are various non-lichenized ascomycetes and basidiomycetes in which this occurs. However, in contrast to Vezdaea, the sexual spores of those other genera can germinate to produce mycelia. There are some more comments in the next reference button![]() .
.
Examples of non-lichenized genera in which conidia are budded off the sexual spores include Ascocoryne, Nectria, Rustroemia (all ascomycetes) and Calocera, Dacrymyces, Exobasidium (basidiomycetes) but the phenomenon hasn't been seen in all species of those genera. If a fungus (lichenized or not) is capable of producing conidia from both the sexual spores and from the mycelium, the conidia from the two sources need not be genetically identical. Amongst both the lichenized and non-lichenized fungi there are species capable of self-fertilization as well as those incapable of self-fertilization. In the latter the nuclei of the sexual spores must, of necessity, contain a genetic mixture from distinct parents. On the other hand, as outlined in the SEXUAL REPRODUCTION CASE STUDY, each cell of the corresponding mycelium contains nuclei from one or both parents, but with no mixing of genes from those nuclei.
Getting the photobiont
In lichens the sexual propagules that are dispersed are the fungal spores. If new thalli are to be formed then those spores must capture the appropriate photobionts and this would be a challenge to at least some degree, but a challenge that has been overcome numerous times since sexual reproduction is known in the majority of lichen species. It is important to emphasize again that in species capable of sexual reproduction this form of reproduction appears to be rare in some species but very common in others. There are various ways in which sexual spores meet the right photobionts. In a few species photobiont cells do occur on apothecia and the ascospores, when released from their asci, may pick up some of those photobiont cells. In such an event both the fungal and photobiont partners are dispersed, so you could call such a combination a symbiotic sexual propagule. Endocarpon, Staurothele and Thelenidia are examples of genera in which photobiont cells are found on apothecia. There is at least one other way in which fungal spores and the right photobiont cells could be dispersed together. Various mites feed on lichens and viable ascospores and photobiont cells have been collected from mite faeces. Obviously the relative concentrations of spores and photobiont cells in a mite's faeces would vary depending on how the mite has been feeding. Nevertheless on many occasions mites are likely to deposit spores together with the right photobiont cells and there's more about mites on the PROPAGULE DISPERSAL page. Despite examples such the ones given in this paragraph it remains a fact that fungal propagules are typically dispersed alone and after landing must find the right photobiont cells![]() .
.
A number of the photobiont species found in lichen partnerships can be found free-living very often. This suggests that such photobionts are able to survive very well as independent organisms and fungi that form lichen partnerships with such species are likely to be able to lichenize fairly easily, given the ready availability of free-living photobiont. On the other hand some common lichen photobiont species are rarely found free-living. Amongst these are species of the genus Trebouxia and some similar genera, grouped together under the label "trebouxioid". This suggests that various photobionts, trebouxioid or otherwise, that are rarely found free-living have some difficulties surviving outside the lichen partnership. That's not to say that they can't survive in the free state for many generations, just that this appears to be rare. For fungal propagules reliant on such photobionts other lichens will sometimes be sources of free-living photobiont cells, at least in the short-term. For example, I've noted above that photobiont cells might escape from a highly saturated lichen thallus. In the right circumstances such liberated cells are able to survive alone for at least some time and are therefore open to capture by the hyphae that have grown out from a nearby fungal propagule. Decaying lichen thalli or fragments and isidia or soredia with moribund fungi are other possible sources of liberated photobiont cells.
Not all mycobionts rely on free-living photobionts. Some mycobionts are parasitic on other lichen thalli, so that a spore of the parasitic mycobiont lands on another lichen thallus and makes use of the photobiont in that thallus. A parasitic mycobiont, depending on species, might completely swamp the host thallus, leading to death of the host fungus, or occupy just part of the host thallus. While various parasites keep the host photobiont Diploschistes muscorum is an example of a parasite in which there is photobiont replacement. This parasite has been seen to start its development on thalli of Cladonia species in which the photobiont is the alga Trebouxia irregularis. Over time the Diploschistes thallus would cover the Cladonia thallus and it is clear that Diploschistes muscorum can use Trebouxia irregularis. However, during the growth of the parasitic thallus the original photobiont is replaced with Trebouxia showmannii so that in a well-established Diploschistes muscorum thallus there is no trace of Trebouxia irregularis. By that time the Diploschistes thallus is leading an independent existence, being parasitic only during establishment. In this example the change of photobiont indicates that Trebouxia showmannii is the ideal photobiont partner but that the fungus can make-do for a while with a non-ideal partner![]() .
.
Xanthoria parietina is another species which, though not parasitic on lichen thalli, can start by associating with a non-ideal photobiont before finding the ideal photobiont, Trebouxia arboricola. Since trebouxioid photobionts are rarely found free-living a hypha growing out from a Xanthoria parietina spore is more likely to come into contact with photobionts other than Trebouxia and the fungus is able to form interim associations with other photobionts. During such interim associations the fungus can survive and grow but there is little differentiation of any sort of thallus and certainly no formation of a Xanthoria-type thallus. Xanthoria parietina ascospores are typically ejected from asci in groups of eight. If several spores germinate their hyphae are likely to grow in different directions, thereby increasing the search area. The hyphae might capture free-living Trebouxia cells or cells within soredia that have come to rest nearby, not necessarily soredia that have been released from a Xanthoria thallus. Thus free-living Trebouxia is not the only source of photobiont. Xanthoria parietina produces neither isidia nor soredia. It may reproduce vegetatively by fragmentation but very often thalli of this species are found with apothecia indicating that high reliance is placed on sexual reproduction. The species is fairly common in many parts of the world so obviously the strategy outlined in the previous few sentences has been very successful![]() .
.
How long can a lonely mycobiont last?
If a mycobiont is reliant on a widespread, free-living photobiont then the chances of a germinating spore finding the photobiont in a short time are good. On the other hand, the chances of finding rarely free-living photobionts in an area poor in decaying thalli or moribund propagules is rather poor. If a spore lands and germinates in such an area, how long can it survive in the non-lichenized state?
An experiment in which mycobiont mycelia were grown in the laboratory and then transplanted to twigs of outdoor oak trees showed that such non-lichenized mycelia could survive for up to a year in natural conditions. The species involved in the experiment were: Anaptychia ciliaris, Physcia tenella, Physconia distorta and Xanthoria parietina. During the year in the field none of the transplanted mycelia showed marked growth but they were still present and hadn't degenerated. Physcia tenella showed some initial steps towards thallus formation. These steps were initiated by the proximity of free-living algae or by soredia of the same species landing on or near the transplanted mycelium. Certainly these results are very interesting and suggest that some mycobionts might be able to persist for a considerable time after spore germination, until the right photobiont turns up. However, there is the question of how well the experimental results carry across to mycobionts in the wild. In the experiment spores were grown on a nutrient-impregnated agar medium. The mycelia were stored in the laboratory at room temperature for a year (with no renewal of the medium) before being placed outdoors. In the wild a spore would need to germinate and establish a viable mycelium while fending off any competitors or predators and putting up with the vagaries of the weather. It is thought that, in general, mycobionts would need to find photobionts fairly soon but the experiment reported above does suggest that at least some mycobionts need not be in a rush to find the right photobionts![]() .
.
It has been suggested that many lichen mycobionts might be able to survive for just a short while as saprotrophs while awaiting the right photobionts but a few species are known to exist long term in either saprotrophic or lichenized form. In such a species it is conceivable that a fungal spore released from the lichenized form could germinate to produce the saprotrophic form. Later, the spores released by that saprotrophic form might capture a photobiont to produce the lichenized form. In this example a non-lichenized 'generation' sits between two lichenized 'generations' and it is feasible that the lichenized form (or the saprotrophic form) might be skipped for several 'generations' until the right combination of circumstances occurred again. The lichen genus Conotrema is an example, with DNA studies showing no differences between some lichenized Conotrema species and some non-lichenized Stictis species. There is more about this in the STICTIS AND CONOTREMA CASE STUDY.
Vegetative with a twist: switching partners
Though a propagule such as an isidium or soredium disperses both partners the new thallus need not continue that exact partnership. Suppose an isidium or soredium is carried some distance and lands near free-living photobiont cells - of a genetically different variant of the same species as in the propagule or perhaps a different species, but closely related to that in the propagule. At the new location the hyphae growing out from the propagule are likely to meet the free-living photobiont cells and, given the similarity between photobionts, the hyphae might be able to capture the free-living cells. If the combination of 'propagule fungus + new photobiont' is better adapted to the new location than is the combination within the propagule the new thallus might develop with photobiont cells of only the new variant or with that variant dominating. In the genus Lepraria sexual reproduction has never been seen and the species reproduce vegetatively via soredia. Studies of Lepraria samples from a variety of Eurasian and American locations has shown that the sort of switching described above occurs in this genus. It has been suggested that switching could give the lichen association benefits somewhat similar to those of recombination via sexual reproduction. For example, a soredium may come to rest in an area where the soredial alga is less efficient in the new light levels than an alga resident in that new area. In this case switching photobionts would be beneficial to the fungus. By associating with a fitter partner the fungus would have a better chance of living in the new environment. Another suggestion is that switching partners may be a way of escaping grazers or parasites. Many lichen grazers or parasites are known and it is also known that a number of lichen secondary metabolites act as deterrents. From the algal viewpoint switching would be beneficial of the new mycobiont produced better deterrents. In a paper comparing sexual reproduction and vegetative reproduction via soredia in two species of Physconia the authors wrote:
We suggest that the main role of the photobiont in soredia is to prolong the survival of the co-propagated fungal hyphae. Depending on the viability of the soredial algae, the soredial fungus can choose between establishing a thallus with the rather few co-propagated alga or with the adjacent and possibly more vital free-living alga
.
Reproduction & dispersal pages on this website |
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)